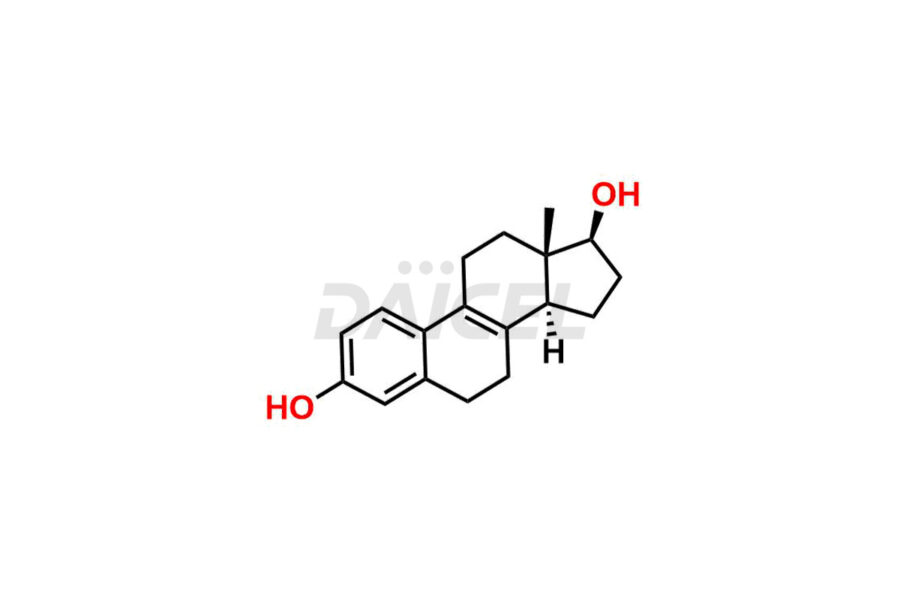
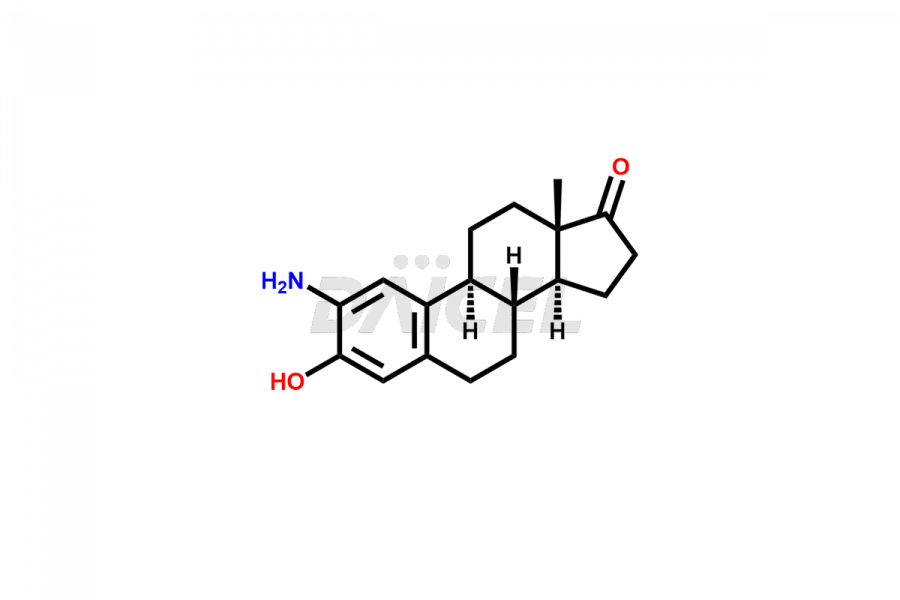
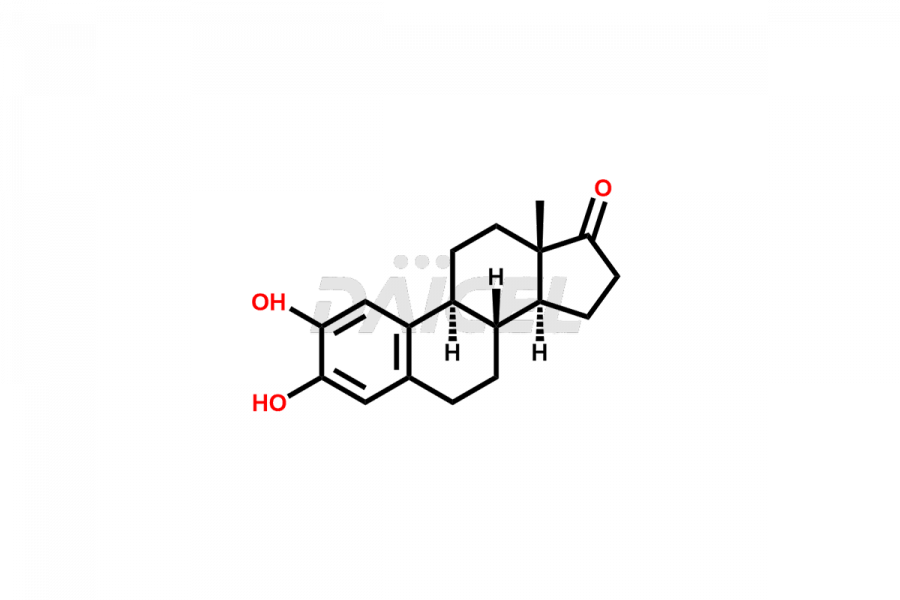
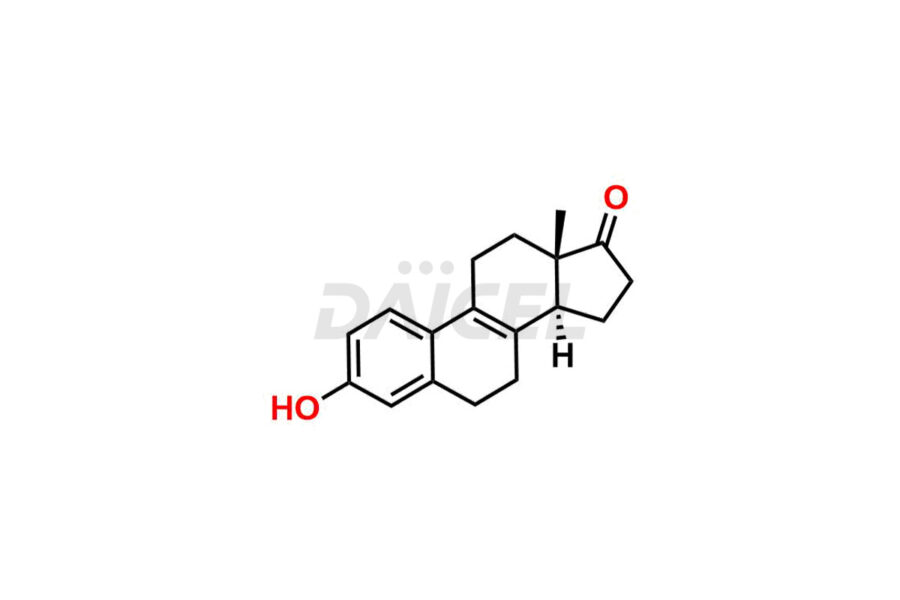
Estrone
General Information
Estrone Impurities and Estrone
Daicel Pharma offers high-quality impurities for Estrone, an active pharmaceutical ingredient. These impurities, including 17 Beta Delta 8,9 Dehydro Estradiol, and D8,9 Dehydro Estrone, play a vital role in assessing Estrone purity, reliability, and safety. Daicel Pharma also offers a customized synthesis of Estrone impurities to cater to client requirements, with worldwide delivery options available.
Therapeutic Estrone [CAS: 53-16-7] is a synthetic form of the estrogen hormone Estrone, which occurs naturally in the body. It is a weak estrogen, a steroid that helps preserve bone density, and has antineoplastic (anti-cancer) properties. Estrone stimulates the production of sex hormone-binding globulin (SHBG) and thyroid-binding globulin (TBG) in the liver. Additionally, it suppresses the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary gland.
Estrone: Use and Commercial Availability
The most common form of estrogen hormone is Estrone, produced in the ovaries and associated with female sexual characteristics development. Estrone is used in hormone replacement therapy (HRT) for managing menopause-related symptoms. It is available under Theelin.
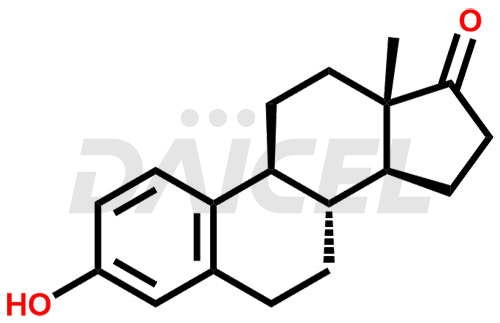
Estrone Structure and Mechanism of Action 
The chemical name of Estrone is 3-Hydroxyestra-1,3,5(10)-trien-17-one. Its chemical formula is C18H22O2, and its molecular weight is approximately 270.4 g/mol.
After diffusing through the cell membranes, Estrone binds to the nuclear estrogen receptor in the reproductive tract, hypothalamus, liver, breast, pituitary gland, and bone. It promotes the transcription of target genes that helps in the functions of the female reproductive system.
Estrone Impurities and Synthesis
Controlling the synthesis, analyzing, and managing impurities in Estrone is crucial for maintaining the purity and safety of the compound. The synthetic process1 involves identifying and understanding the potential pathways that can lead to the formation of impurities. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry help analyze and quantify these impurities in Estrone samples. Strict control measures are implemented during production to minimize impurity formation and maintain the quality of Estrone. Regular monitoring and adherence to regulatory guidelines ensure the efficacy and safety of Estrone for its intended applications.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for Estrone impurity standards, including 17 Beta Delta 8,9 Dehydro Estradiol and D8,9 Dehydro Estrone. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Estrone impurities or degradation products. Every delivery has a complete characterization report.
References
FAQ's
References
- Rosenkranz, G.; Mancera, O.; Sondheimer, Franz; Djerassi, Carl, Steroids. LXXXI. Transformation of sapogenins to androgens and estrogens. Beckmann rearrangement of Δ16-20-oxo steroids, Journal of Organic Chemistry, Volume: 21, Pages: 520-2, 1956
- Attal, Joe; Hendeles, S. M.; Eik-Nes, Kristen B., Determination of free estrone in blood plasma by gas-phase chromatography with electron capture detection, Analytical Biochemistry, Volume: 20, Issue: 3, Pages: 394-410, 1967
Frequently Asked Questions
What are the potential risks associated with unidentified or uncontrolled Estrone impurities?
Uncontrolled or unidentified impurities in Estrone pose risks such as unknown toxicity, potential drug interactions, and compromised therapeutic efficacy, highlighting the importance of their identification and control.
Can Estrone impurities result in changes to the drug's physical properties?
Impurities in Estrone can potentially lead to changes in the drug's physical properties, such as color, odor, or stability, affecting its acceptability and patient compliance.
Can Estrone impurities affect its dissolution rate or bioequivalence?
Impurities in Estrone can affect its dissolution rate, which may impact its bioavailability and bioequivalence, raising concerns about therapeutic effectiveness.
How should Estrone impurities be stored in terms of temperature?
Estrone impurities require storage at a controlled room temperature, between 2-8 °C, or according to the Certificate of Analysis (CoA) specifications.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.