Doxazosin
General Information
Doxazosin Impurities and Doxazosin
Daicel Pharma specializes in offering high-quality impurities for Doxazosin, an active pharmaceutical ingredient. The impurity, Doxazosin Mesylate Related Compound, plays a vital role in assessing Doxazosin’s purity, reliability, and safety. Daicel Pharma also offers a customized synthesis of Doxazosin impurities to cater to client requirements, with worldwide delivery options available.
Doxazosin [CAS: 74191-85-8] exhibits antihypertensive and antineoplastic properties. The quinazoline compound selectively inhibits alpha-1 adrenergic receptors. It treats hypertension and benign prostatic hypertrophy as a nonselective alpha-blocker.
Doxazosin: Use and Commercial Availability
Doxazosin is a medication that offers therapeutic benefits for several conditions. Derived from quinazoline, it functions as a competitive alpha1-antagonist. It manages benign prostatic hyperplasia, ureteral stones, and hypertension. Symptoms associated with benign prostatic hypertrophy, including urinary frequency, urgency, and nocturia, are relieved with Doxazosin. It can be prescribed alone for hypertension control or with other antihypertensive agents. Doxazosin is available under the brand names Cardura and Cardura XL.
Doxazosin Structure and Mechanism of Action 
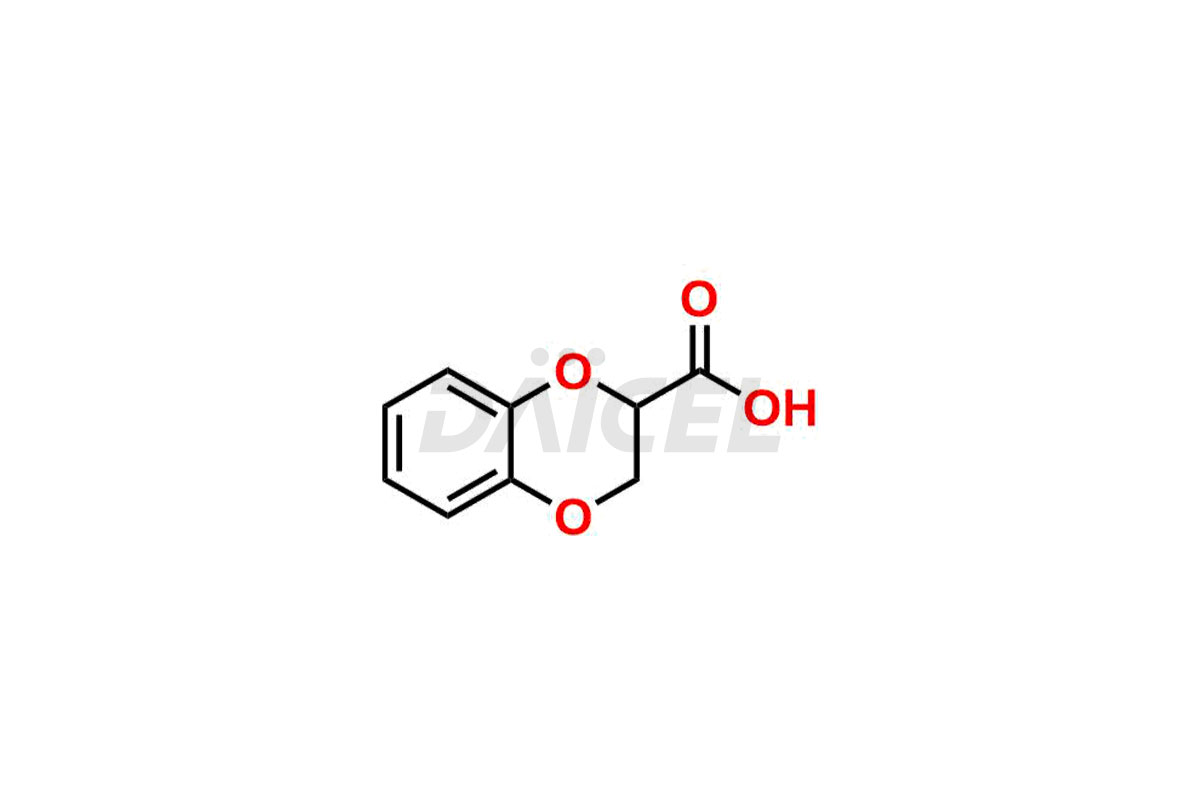
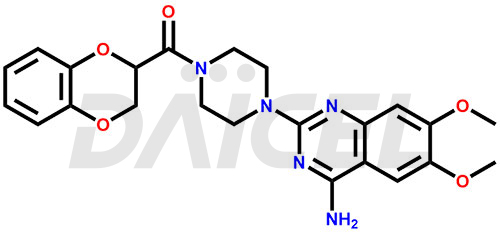
The chemical name of Doxazosin is [4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2,3-dihydro-1,4-benzodioxin-2-yl)methanone. Its chemical formula is C23H25N5O5, and its molecular weight is approximately 451.5 g/mol.
Doxazosin competitively antagonizes the pressor effects of phenylephrine, an alpha1 agonist. It also opposes the systolic pressor effect of norepinephrine.
Doxazosin Impurities and Synthesis
Doxazosin impurities refer to the byproducts or related substances that may be present in the Doxazosin drug. The synthesis of Doxazosin impurities involves the preparation of these unintended compounds during the manufacturing process1. Analysis and control of Doxazosin impurities are crucial to ensure the drug’s quality, safety, and efficacy. Various analytical techniques, such as high-performance liquid chromatography (HPLC), help identify and quantify these impurities. Stringent quality control measures help to limit the presence of Doxazosin impurities within acceptable limits, adhering to regulatory guidelines and ensuring the purity of Doxazosin.
Daicel Pharma offers a comprehensive Certificate of Analysis (CoA) for the Doxazosin impurity standard, such as Doxazosin Mesylate Related Compound. They generate from an analytical facility that complies with cGMP standards. The CoA provides a detailed characterization report with data obtained through techniques such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2 analysis. We give additional data like 13C-DEPT upon request. Daicel Pharma synthesizes unknown Doxazosin impurities or degradation products, and labeled compounds, to evaluate the efficacy of generic Doxazosin. Also, Doxazosin-D8, a deuterium-labeled Doxazosin standard, is available for bio-analytical research, including BA/BE studies. Every delivery has a complete characterization report.
References
FAQ's
References
- Campbell, Simon Fraser, Antihypertensive 4-amino-2-[4-(1,4-benzodioxan-2-carbonyl) piperazin-1-yl or homopiperazin-1-yl]quinazolines, Pfizer Corp., Panama, US4188390A, February 12, 1980
- Cowlishaw, M. G.; Sharman, J. R., Doxazosin determination by high-performance liquid chromatography using fluorescence detection, Journal of Chromatography, Biomedical Applications, Volume: 344, Pages: 403-7, 1985
Frequently Asked Questions
What are the potential sources of impurities in Doxazosin?
The potential sources of impurities in Doxazosin include raw materials, reagents, solvents, and reaction intermediates used in the manufacturing process.
Can Doxazosin impurities affect the effectiveness of the medication?
Depending on the nature and concentration, impurities in Doxazosin can affect the drug's efficacy, stability, and safety.
What steps help ensure the control of Doxazosin impurities during drug development?
During drug development, extensive studies help identify and characterize impurities in Doxazosin, establish acceptable limits, and implement suitable control strategies.
What are the temperature conditions required to store Doxazosin impurities?
The recommended temperature for storing impurities in Doxazosin is a controlled room temperature, typically between 2-8 °C. Alternatively, the specific temperature indicated on the Certificate of Analysis (CoA) is required for proper storage.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.