Desogestrel
General Information
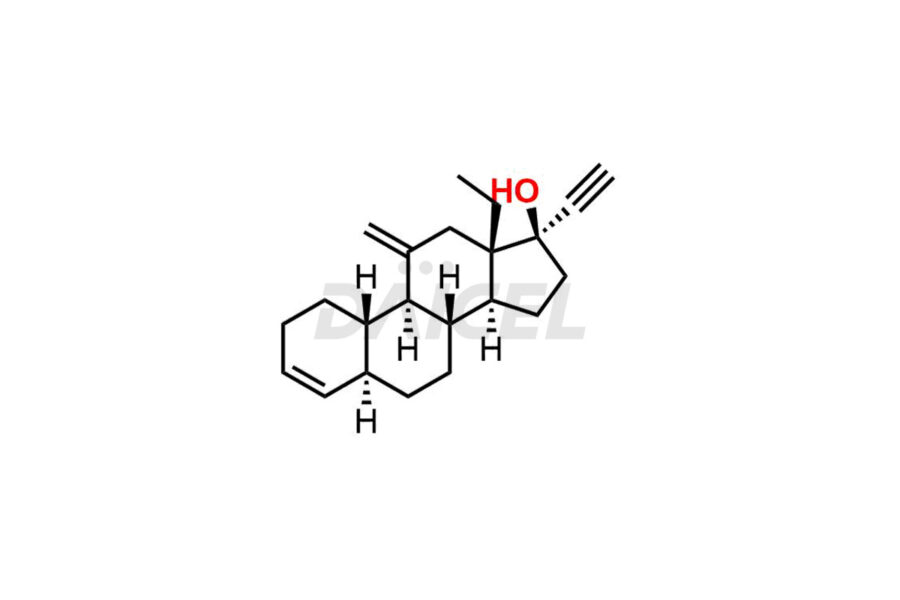
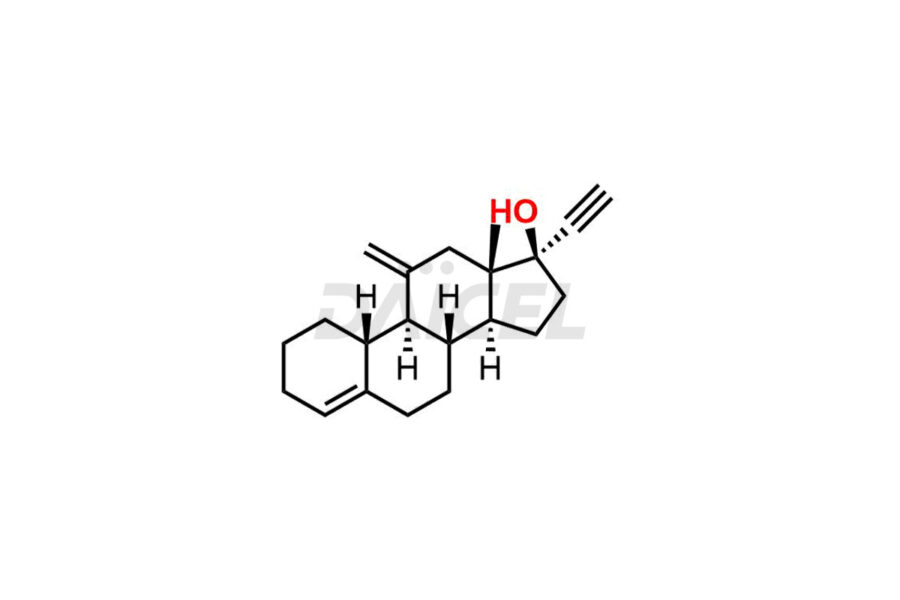
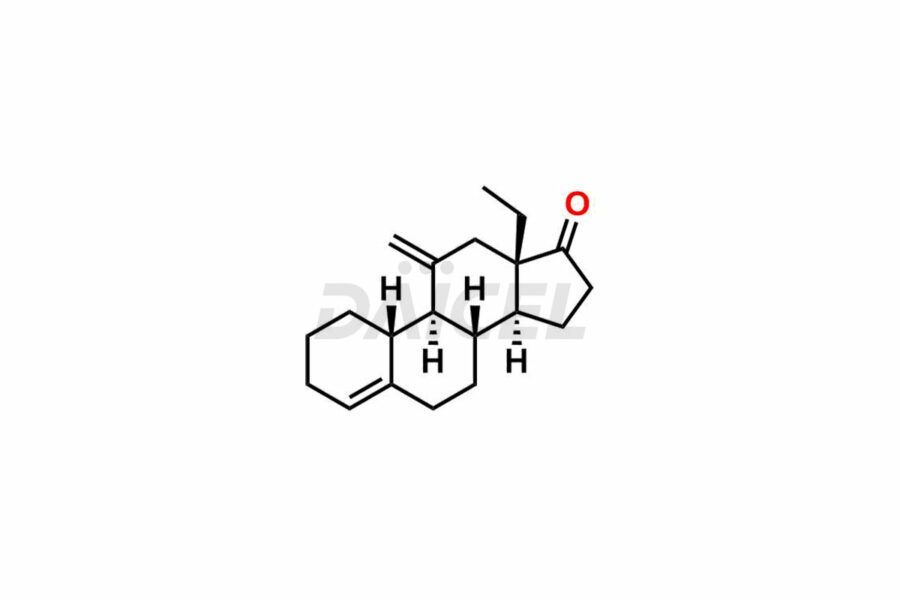
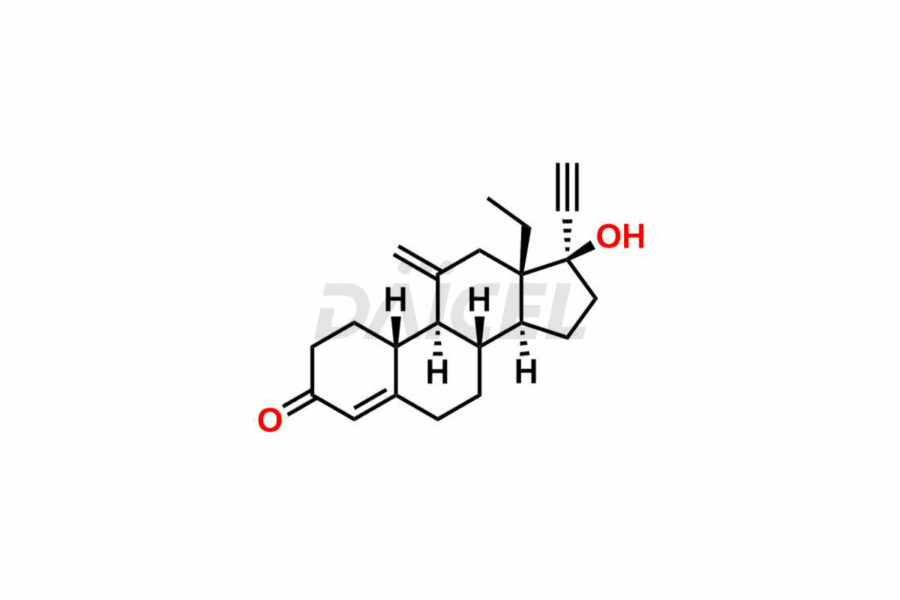
Desogestrel Impurities and Desogestrel
Daicel Pharma synthesizes high-quality Desogestrel impurities, such as Desogestrel Impurity A, Desogestrel Impurity B, Desogestrel Impurity C, Desogestrel impurity D, and Desogestrel impurity E, crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient, Desogestrel. Moreover, Daicel Pharma offers custom synthesis of Desogestrel impurities and delivers them globally.
Desogestrel [CAS: 54024-22-5] is a synthetic progestogen similar in structure to levonorgestrel. It activates progesterone hormone receptors. It acts as a contraceptive and a hormone replacementtherapy.
Desogestrel: Use and Commercial Availability
Desogestrel is available in combination with ethinyl estradiol in the US as an oral contraceptive. However, it is sold under various brand names globally, such as Bekyree, Cyclessa, Desogen, Emoquette, Enskyce, Isibloom, Kalliga, Kariva, Kimidess, Mircette, Ortho-Cept, Pimtrea, Simliya, Velivet, Viorele, Volnea, among others.
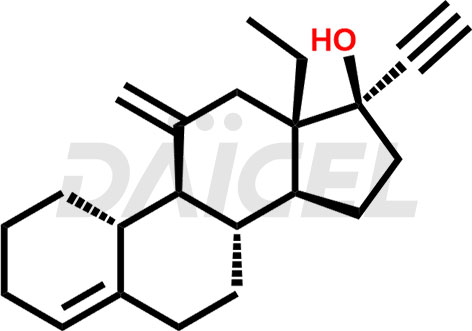
Desogestrel Structure and Mechanism of Action
The chemical structure of Desogestrel is 13-Ethyl-11-methylene-18,19-dinor-17α-4-pregnen-20-yn-17-ol. Its chemical formula is C22H30O, and its molecular weight is approximately 310.5g/mol.
Desogestrel inhibits ovulation. It causes changes in cervical mucus that prevents sperm entry into the uterus. Further, changes in the endometrium reduce the likelihood of implantation. Oral contraceptives suppress gonadotropins.
Desogestrel Impurities and Synthesis
During the manufacturing process1, impurities may form in Desogestrel due to various reasons such as incomplete reactions, side reactions, degradation, and contamination. The presence of these impurities in Desogestrel can affect its efficacy and safety. Therefore, control of impurity formation and their levels in the final product is essential.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Desogestrel impurity standards, including Desogestrel Impurity A, Desogestrel Impurity B, Desogestrel Impurity C, Desogestrel impurity D, and Desogestrel impurity E. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report. Daicel has the technology and expertise to prepare any unknown Desogestrel impurity or degradation product. Daicel also provides labeled compounds3 to quantify the efficacy of generic Desogestrel. Daicel offers highly pure isotope-labeled standards of Desogestrel for bioanalytical research and BA/BE studies with isotope data in CoA.
References
FAQ's
References
- Ring, Sven; Teichmueller, Gerhard; Weber, Gisela; Schwarz, Sigfrid; Erhart, Bernd; Undeutsch, Bernd; Raethe, Harald; Moellmann, Peter; Pfeiffer, Carmen; Palme, Hans-Joachim, “Steroid intermediates and method of their preparation”, Jenapharm GmbH, US5831104A, November 3, 1998.
- Smilde, A. K.; Bruins, C. H. P.; Doornbos, D. A.; Vink, J., “Optimization of the reversed-phase high-performance liquid chromatographic separation of synthetic estrogenic and progestogenic steroids using the multi-criteria decision making method”, Journal of Chromatography, Volume: 410, Issue: 1, Pages: 1-12, 1987
- Griffith, David R.; Wacker, Lukas; Gschwend, Philip M.; Eglinton, Timothy I., “Carbon isotopic (13C and 14C) composition of synthetic estrogens and progestogens”, Rapid Communications in Mass Spectrometry, Volume: 26, Issue: 22, Pages: 2619-2626, 2012
Frequently Asked Questions
How are Desogestrel impurities detected?
Impurities in Desogestrel are detected using analytical methods such as high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS).
How are Desogestrel impurities controlled during manufacturing?
The control of Impurities in Desogestrel during manufacturing is through various methods such as process optimization, purification steps, and quality control testing.
How are Desogestrel impurities removed through purification steps?
Desogestrel impurities are removed through various purification steps such as crystallization, chromatography, or filtration.
What are the storage conditions for Desogestrel to prevent impurities?
Desogestrel should be stored in a cool, dry place away from light and moisture to prevent the formation of impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.