LOAD MORE
You're viewed 9 of 14 products
Daicel Pharma synthesizes high-quality Cyclophosphamide impurities, 3-aminopropyl hydrogen bis(2-chloroethyl)phosphoramidate hydrochloride, Cyclophosphamide Related Impurity (Closed Ring Ethanol Aduct), Cyclophosphamide Related Impurity (Open Ring Ethanol Aduct) and Dihydroxybenzyl cyclophosphamide, which is crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Cyclophosphamide. Moreover, Daicel Pharma offers custom synthesis of Cyclophosphamide impurities and delivers them globally.
Cyclophosphamide [CAS: 50-18-0] is an antineoplastic drug related to nitrogen mustards. It is an alkylating drug for treating malignant diseases and minimal change nephrotic syndrome in pediatric patients. Cyclophosphamide is a potent immunosuppressant by reducing T-regulatory cells at low doses.
Cyclophosphamide is US FDA recommended for treating malignant lymphomas, such as lymphocytic lymphoma, Burkitt lymphoma, Hodgkin’s disease, small lymphocytic lymphoma, and multiple myeloma. It is for treating retinoblastoma, breast cancer, ovarian adenocarcinomas, disseminated neuroblastomas, and minimal change nephrotic syndrome in pediatric patients. Cyclophosphamide helps treat autoimmune diseases like multiple sclerosis as a potent immunosuppressive agent. Cyclophosphamide is available under trade names such as Cytoxan, Lyophilized Cytoxan, and Neosar.
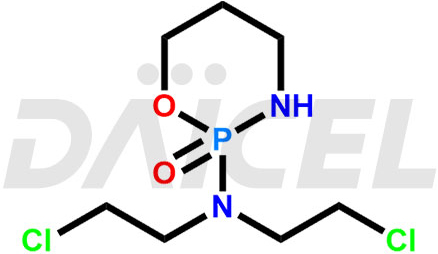
The chemical name of Cyclophosphamide is 2-[Bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide. Its chemical formula is C7H15Cl2N2O2P, and its molecular weight is approximately 261.09 g/mol.
Cyclophosphamide involves cross-linking of tumor cell DNA.
Cyclophosphamide can contain various impurities1 like related compounds, degradation products, etc., which may form during the synthesis or storage of the drug. These impurities can affect the purity, efficacy, and safety of the drug and have to be monitored during the manufacturing and storage processes.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Cyclophosphamide impurity standards, 3-aminopropyl hydrogen bis(2-chloroethyl)phosphoramidate hydrochloride, Cyclophosphamide Related Impurity (Closed Ring Ethanol Aduct), Cyclophosphamide Related Impurity (Open Ring Ethanol Aduct) and Dihydroxybenzyl cyclophosphamide. The CoA includes complete characterization data, such as 1H NMR, 13C NMR2, IR, MASS, and HPLC purity. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Cyclophosphamide impurity or degradation product.
Cyclophosphamide can undergo degradation and form impurities when exposed to various stress conditions, such as acid or base hydrolysis, oxidation, and heat.
Cyclophosphamide has a characteristic UV absorption spectrum, which allows it to be detected and quantified using a UV detector. The concentration of Cyclophosphamide and its impurities are determined by comparing the UV absorption of the sample to that of known standards.
The impurities can affect the quality of Cyclophosphamide by reducing its potency, altering its pharmacokinetics or pharmacodynamics, causing unwanted side effects, or even posing a risk to patient safety.
The control of Cyclophosphamide impurities is regulated by various international regulatory bodies such as the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the US Food and Drug Administration (FDA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.