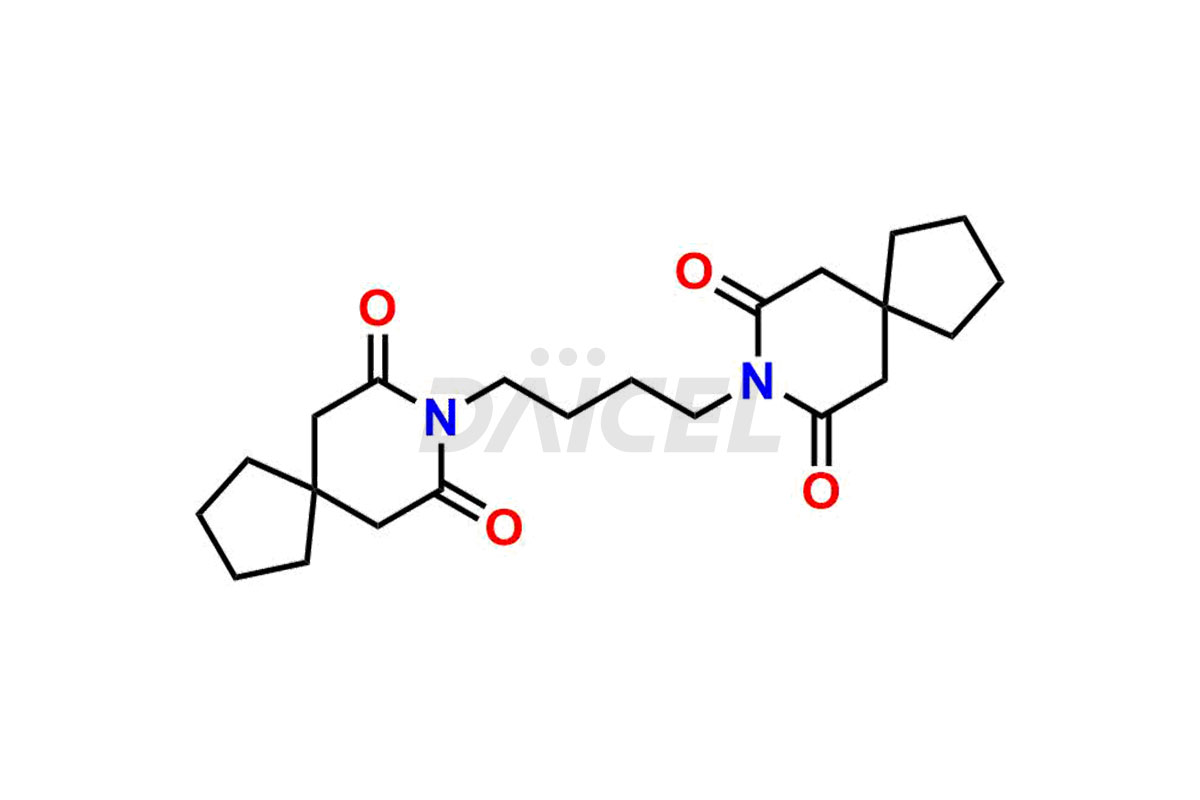
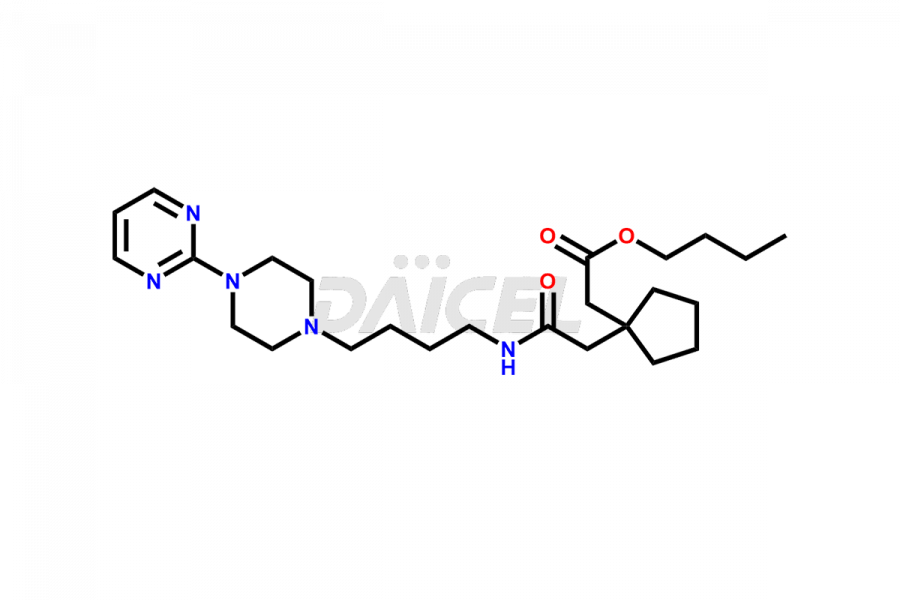
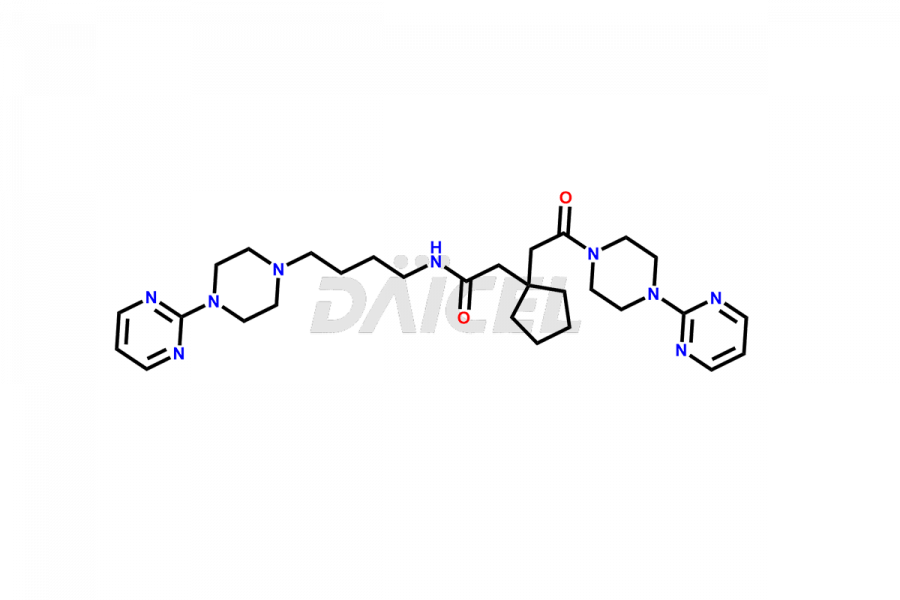
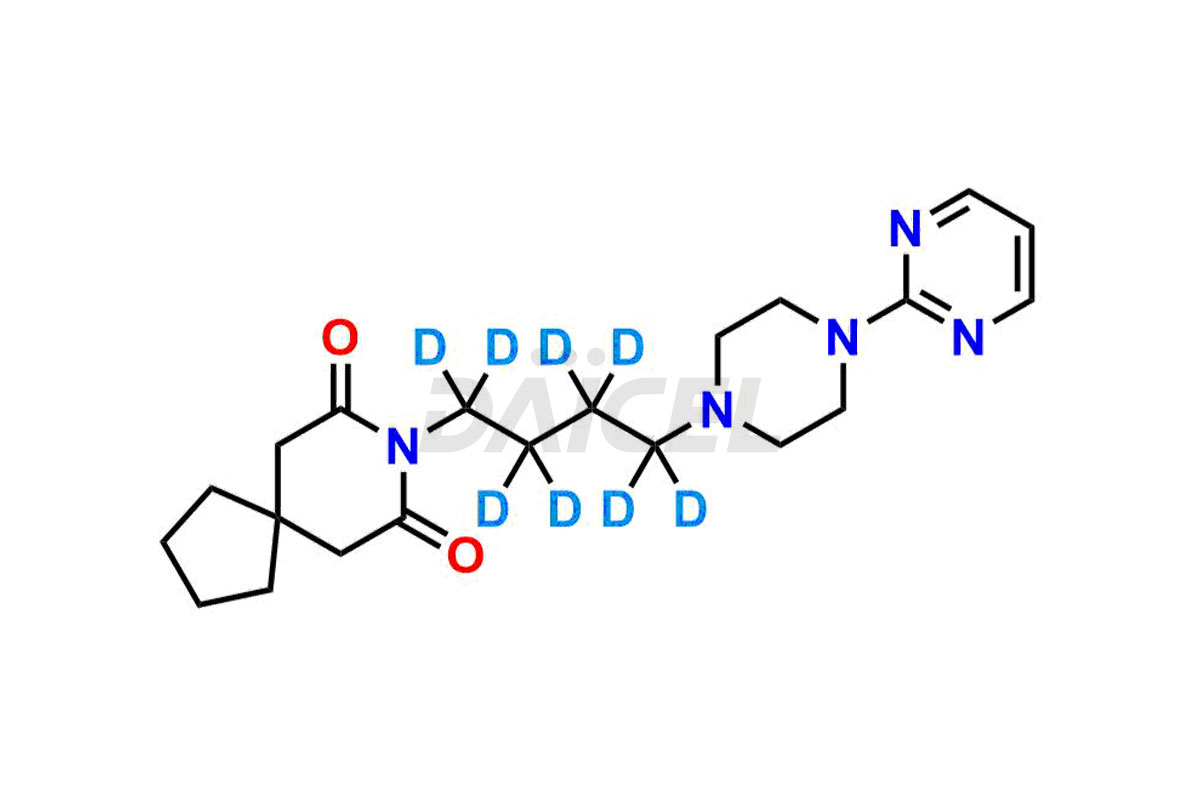
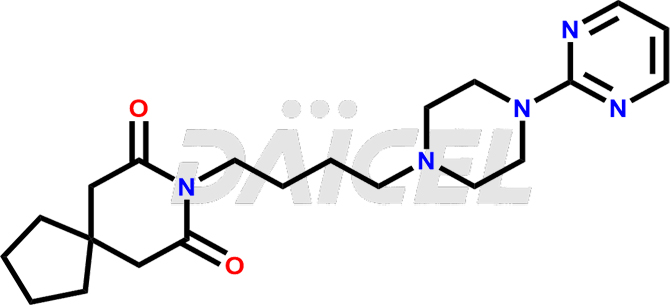
Buspirone
General Information
Buspirone Impurities and Buspirone
Daicel Pharma synthesizes Buspirone impurities of exceptional quality, such as Buspirone EP Impurity N and Buspirone N-Oxide. These impurities are crucial to assess the purity, reliability, and safety of Buspirone, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Buspirone impurities to meet clients’ demands for worldwide delivery.
Buspirone [CAS: 36505-84-7] is a medication used to manage general anxiety disorders and alleviate anxiety symptoms. It is a psychoactive drug and a partial agonist at serotonin 5-HT1A autoreceptors. Buspirone is also considered an antidepressant.
Buspirone: Use and Commercial Availability
Buspirone is a drug to treat generalized anxiety disorder (GAD) and provides short-term relief for anxiety symptoms. It manages anxiety disorders. The drug is available under the trade name Buspar.
Buspirone Structure and Mechanism of Action 
The chemical name of Buspirone is 8-[4-[4-(2-Pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione. Its chemical formula is C21H31N5O2, and its molecular weight is approximately 385.5 g/mol.
The mechanism of action of Buspirone is unknown.
Buspirone Impurities and Synthesis
Buspirone impurities are unintended substances that generate during the synthesis1, manufacturing, or storage of the drug substance. These impurities can affect the drug quality, safety, and efficacy. The main impurities found in Buspirone are related to the starting materials used in its synthesis. Therefore, it is necessary to control these impurities to ensure the quality and safety of the drug product.
Daicel Pharma offers a Certificate of Analysis (CoA) for Buspirone impurity standards, such as Buspirone EP Impurity N and Buspirone N-Oxide, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data2 from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Buspirone impurities or degradation products and labeled compounds to assess the effectiveness of generic Buspirone. We also offer Buspirone-D8, a deuterium-labeled Buspirone standard useful in bio-analytical research, such as BA/BE studies. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Wu, Yao Hua; Rayburn, James W., N-(Heteroarcyclic)Piperazinylalkyl-Azaspiroalkanediones, Mead Johnson and Co., United States, US3717634A, February 20, 1973
- Gammans, R. E.; Kerns, E. H.; Bullen, W. W., Capillary gas chromatographic-mass spectrometric determination of buspirone in plasma, Journal of Chromatography, Biomedical Applications, Volume: 345, Issue: 2, Pages: 285-97, 1985
- Diaz-Marot, A.; Puigdellivol, E.; Salvatella, C.; Comellas, L.; Gassiot, M., Determination of buspirone and 1-(2-pyrimidinyl)piperazine in plasma samples by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 490, Issue: 2, Pages: 470-3, 1989
Frequently Asked Questions
How are the impurities in Buspirone controlled?
Impurities in Buspirone can be controlled by using high-quality raw materials, optimizing the synthesis process, and implementing appropriate storage and handling conditions. Analytical methods help detect and quantify impurities in the drug substance and product.
How are impurities in Buspirone monitored during the manufacturing process?
Impurities in Buspirone are monitored at various stages of the manufacturing process through in-process controls, such as sampling and testing of intermediate products.
Which solvent helps in the analysis of Buspirone impurities?
Methanol is the solvent used in analyzing many impurities in Buspirone.
What are the temperature conditions required to store Buspirone impurities?
Buspirone impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.